NCERT Solutions for Science Class 9 Chapter 4
Chapter 4: Structure of the Atom
Intext Exercise 1
1. What are canal rays?
Answer: Canal rays are beams of positively charged ions. It was discovered in 1886 by Goldstein. Canal rays are also known as anode rays.
2. If an atom contains one electron and one proton, will it carry any charge or not?
Answer: An atom carrying one electron and one proton will carry no charge as the negatively charged particle will combine with the positively charged particles and the overall magnitude of the atom will be zero.
3. On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer: According to Thomson’s model of the atom:
1. An atom consists of both negatively and positively charged particles.
2. The negatively charged particles are embedded in the positively charged sphere.
3. These negative and positive charges are equal in magnitude.
4. They counterbalance each other’s effect and make an atom neutral.
4. On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Answer: According to Rutherford’s model of an atom, protons are present in the nucleus of an atom.
5. Draw a sketch of Bohr’s model of an atom with three shells.
Answer:
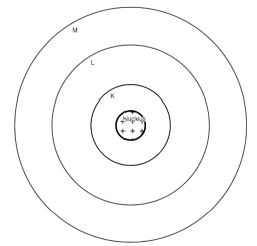
6. Name the three subatomic particles of an atom.
Answer: Three subatomic particles of an atom are as follows:
i. Proton
ii. Electron
iii. Neutron.
7. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer:
Number of protons in Helium Atom =2
Atomic Mass=Number of Proton+Number of Neutrons
4=2+Number of Neutrons
Number of Neutrons =4-2=2
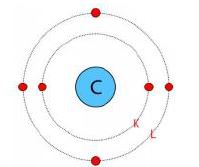
8. Write the distribution of electrons in carbon and sodium atoms?
Answer: Atomic number of carbon =6=Number of protns=Number of electrons
The distribution of electrons in carbon atom is given by:
First orbit of K-shell = 2 electrons
Second orbit of L-shell = 4 electrons
Or, we can write the distribution of electrons in a carbon atom as 2, 4
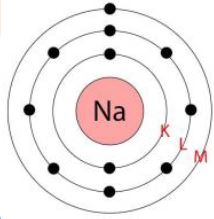
Atomic number of sodium = 11= Number of protons = Number of electrons
The distribution of electrons in sodium atom is given by:
First orbit of K-shell= 2 electrons
Second orbit of L-shell = 8 electrons
Thirs orbit or L-shell = 8 electrons
Third orbit or M-shell = 1 electron
Or, we can write the distribution of electrons in a sodium atom as 2, 8, 1
9. If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer: Maximum number of electron in K-shell =2
Maximum number of electron in L-shell =8
If K and L -shell of an atom are full,
then the total number of electrons in the atom would be (2+8)=10 electrons.
10. How will you find the valency of chlorine, sulphur and magnesium?
Answer: If the number of electrons in the outermost shell is less than 4 then,
Valency of an atom = number of electrons in the outermost shell of the atom.
1. In the case of magnesium,
Thus, the valency of magnesium = 2
If the number of electrons in the outermost shell is less than 4 then,
Valency of an atom = 8 – Number of electrons in the outermost shell.
2. In case of sulphur,
The valency of sulphur = 8 − 6 = 2
3. In case of chlorine,
The valency of chlorine = 8 −7 = 1
11. If the number of electrons in an atom is 8 and the number of protons are also 8, then
(i) What is the atomic number of the atom and (ii) What is the charge on the atom?
Answer:
1. The atomic number = number of protons.
Hence, the atomic number of the atom is 8 .
2. Since the number of both electrons and protons is equal, therefore, the charge on
the atom is 0 i.e., it is a neutral atom.
12. With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Answer: Mass number = Number of protons + Number of neutrons
Mass number of \(O_2=8+8=16\)
Mass number of \(S=16+16=32\)
13. For the symbols H, D and T tabulate three sub-atomic particles found in each of them.
| Symbol | Electron | Proton | Neutron |
| H | 1 | 1 | 0 |
| D | 1 | 1 | 1 |
| T | 1 | 1 | 2 |
14. Write the electronic configuration of any one pair of isotopes and isobars.
Answer:
Two isotopes of carbon are:
(i) \(^{12}_6C\)
(ii) \(^{14}_6C\)
The electronic configuration of \(^{12}_6C\) is \(2,\space 4\)
The eletronic configuration of \(^{14}_6C\) is \(2,\space 4\)
Two isobars of carbon are:
(i) \(^{40}_{20}Ca\)
(ii) \(^{40}_{18}Ar\)
The electronic configuration of \(^{40}_{20}Ca\) is 2, 8, 8, 2.
The electronic configuration of \(^{40}_{18}Ar\) is 2, 8, 8.
Exercise Questions:
1. Compare the properties of electrons, protons and neutrons.
Answer: The difference between electron, proton and neutrons are as follows:
| Electron | Proton | Neutron |
| It is present outside of the nucleus of an atom. | It is present inside the nucleus of an atom. | It is present inside the nucleus of an atom. |
| Carry negative charge. | Carry positive charge | It is neutral. |
| Its weight is negligible. | It weighs around 2000 times as mass of electrons. | Weight is the same as a proton. |
2. What are the limitations of J.J. Thomson’s model of the atom?
Answer: Limitations of J.J. Thomson’s model of the atom.
- It fails to explain the stability of an atom.
- It doesn’t talk about the nucleus of an atom.
- It failed to explain the reason for positive and negative charges binding together.
- It also doesn’t explain Rutherford’s model.
3. What are the limitations of Rutherford’s model of the atom?
Answer: Rutherford’s model of the atom fails to explain the stability of an atom. He argued that electrons move in a circular path called the orbit. The Revolution of electrons in the orbit will radiate energy which will make the atom unstable and electrons will fall inside the nucleus. But in reality this is not the case and Rutherford’s model fails to explain the reason for the same.
4. Describe Bohr’s model of the atom.
Answer:
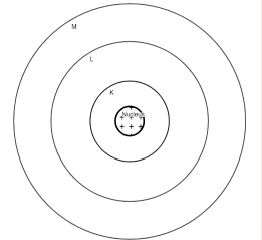
5. Compare all the proposed models of an atom given in this chapter.
Answer: Comparison of all models of an atom are given below:
| Thomson’s Model | Rutherford’s Model | Bohr’s Model |
| According to this mode positively and negatively charged ions are embedded throughout the atom. | This model explained that all the positive ions are embedded in the nucleus and negative ions revolve around it. | Electron or negatively charged particles move around in a fixed circular path called the orbit. There is no energy generation in the electron revolution. |
6. Summarize the rules for the writing of the distribution of electrons in various shells for the first eighteen elements.
Answer: For the first eighteen elements, the rules for writing the distribution of electrons in various shells are as follows:
(i) The maximum number of electrons that a shell can accommondate is detemined by the formula ‘\(2n^2\)’, where ‘\(n\)’ us the orbit numbe (\(n=1,2,3…..\)).
(ii) The maximum number of electrons present in an orbit of \(n=1\) is given by \(2n^2=2(1)^2=2\)
Again for second orbit, it is \(2n^2=2(2)^2=2\times4=8\)
For third orbit, it is \(2n^2=2(3)^2=2\times9=18\)
(iii) The outermost orbit can hold a maximum of eight electrons.
(iv) Shells are filled with electrons in a stepwise manner, with the inner shells being filled first, followed by the outer shells.
7. Define valency by taking examples of silicon and oxygen.
Ans: Valency of an element is defined as the number of electrons in its outermost
shell.
In the case of silicon,
Outermost shell electron = 4
If the number of electrons in the outermost shell of the atom of an element is less
than or equal to 4 ,
Valency of an atom = number of electrons in the outermost shell
Thus, the valency of silicon = 4 .
In the case of oxygen,
Outermost shell electron = 6
If the number of electrons in the outermost shell of the atom of an element is
greater than 4 ,
Then, the valency of an atom = 8 – Number of electrons in the outermost shell.
Thus, the valency of oxygen is (8-6)=2.
8. Explain with examples.
i. Atomic number
ii. Mass number,
iii. Isotopes
iv. Isobars.
Give any two uses of isotopes.
Answer:
i. Atomic number: Total number of protons present in the atom of an element is
called the atomic number of that element.
Example: Oxygen has 8 protons. Thus, the atomic number of Oxygen is 8 .
ii. Mass number: The sum of the number of protons and neutrons present in the
atom of an element is called the mass number.
Example: Oxygen has 8 protons and 8 neutrons.
Therefore the mass number of oxygen is 8+8=16
iii. Isotopes: Isotopes are atoms of the same element having the same atomic
number, but different mass numbers.
Example : Three isotopes of Hydrogen are:
(1) protium \((^1H)\)
(2) Deuterium \((^2_1H)\)
(3) Tritium \((^3_1H)\)
(iv) Isobars: Isobars are atoms with the same mass number but different atomic numbers, i.e., isobars are atoms with the same mass number from different elements.
Example: \(^{40}_{20}Ca\) and \(^{40}{18}Ar\) are two isotops.
9. Na+ has completely filled K and L shells. Explain.
Answer: Atomic number of Na = 11 = Total number of electrons
The electronic configuration of Na = 2, 8, 1.
The electronic configuration of Na+ ion = 2 (K-shell), 8 (L-shell)
Thereby Na+ ion has completely filling K and L shells.
10. If bromine atom is available in the form of, say, two isotipes \(^{79}_{35}Br(49.75)\) and \(^{18}_35}Br(503%)\) calculate the average atomic mass of bromine atom.
Answer: Average atomic mass of Bromine atom
\(=\frac{[79\times49.7+81\times50.3]}{100}\)
\(=\frac{3926.3+4074.3}{100}\)
\(=\frac{8000.6}{100}=80.006≈80u\)
11. Theaverage atomic mass of a sample of an element \(X\) is \(16.2u\). What are the percentages of isotopes \(^{16}_{8}X\) and \(^{18}_8X\) in the sample?
Answer: Average atomic mass of an element \(X=16.2\space u\)
Let percentage of isotope \(^{18}_8X\) is \(y%\).
Thus, the percentage of isotpe \(^{18}_8X\) is \((100-y)%\)
Average atomic mass of element \(X=\)[Atomic mass of \(^{18}_8X\timespercentage+Atomic\space mass\) of \(^{16}_8X\timespercentage\)]
\(16.2=[18\times y%+16\times(100-y)%\)
\(16.2=[18\times\frac{y}{100}+16\times\frac{(100-y)}{100}]\)
\(16.2\times100=[18y+1600-16y]\)
\(1620=2y+1600\)
\(1620-1600=2y\)
\(20=2y\)
\(y=\frac{20}{2}\)
\(y=10\)
Thus, percentage of \(^{16}_8X\) is \($10%$\)
Percentage of \(^{16}_8X\) is \((100-10)=90%\)
12. If Z = 3, what would be the valency of the element? Also, name the element.
Answer: Atomic number = Z = 3.
Its electronic configuration is 2, 1.
Hence, the valency of the element is 1
(Since the outermost shell has only one electron).
Therefore, the element with Z = 3 is lithium (Li).
13. Composition of the nuclei of two atomic species X and Y are given as under X Y
Answer:
| X | Y | |
| Protons | 6 | 6 |
| Neutrons | 6 | 8 |
Give the mass numbers of X and Y. What is the relation between the two species?
Answer: Mass number = Number of protons + Number of neutrons
Mass number of X =6 +6 =12
Mass number = Number of protons + Number of neutrons
Mass number of Y =6 + 8 = 14
Atomic number = Number of protons
The atomic number of X= 6 = Atomic number of Y
These two atomic species X and Y have the same atomic number, but different mass numbers. Hence, they are isotopes.
14. For the following statements, write T for ‘True’ and F for ‘False’.
a. J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
Answer: False
b. A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
Answer: False
c. The mass of an electron is about 1/2000 times that of the proton.
Answer: True
d. An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer: False
15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
a. Atomic nucleus
b. Electron
c. Proton
d. Neutron
Answer: (a) Atomic Nucleus
16. Isotopes of an element have
a. The same physical properties
b. Different chemical properties
c. Different number of neutrons
d. Different atomic numbers
Answer: (c) Different numbers of neutrons.
Isotopes are atoms of the same element having the same atomic number, but different mass numbers.
17. Number of valence electrons in \(Cl^{-1}\)ion is:
a. 16
b. 8
c. 17
d. 18
Answer: Atomic number of \(Cl = 17\)
Electronic configuration of \(Cl = 2, 8, 7\)
Electronic configuration of \(Cl^{-1}\) ion =2, 8, 8\)
Thus, the number of valence electrons in \(Cl^{-1}\) ion \(= 8\)
18. Which one of the following is a correct electronic configuration of sodium?
a. 2, 8
b. 8, 2, 1
c. 2, 1, 8
d. 2, 8, 1
Answer: Atomic number of sodium = Number of electrons
So, electronic configuration of sodium = 2, 8, 1.
19. Complete the following table.
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Name of the atomic species |
| 9 | – | 10 | – | – | – |
| 16 | 32 | – | – | – | Sulphur |
| – | 24 | – | 12 | – | – |
| – | 2 | – | 1 | – | – |
| – | 1 | 0 | 1 | 1 | – |
Answer:
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Name of the atomic species |
| 9 | 19 | 10 | 9 | 9 | Fluorine |
| 16 | 32 | 16 | 16 | 16 | Sulphur |
| 12 | 24 | 12 | 12 | 12 | Magnesium |
| 1 | 2 | 1 | 1 | 1 | Deuterium |
| 1 | 1 | 0 | 1 | 1 | Protonium |






